Determination of AD-related biomarkers
In clinical practice the mainstream for the determination of protein biomarkers in human fluids is represented by immunoassay methods based on kits for ELISA (enzyme-linked immunosorbent assay), supplied by highly valuable companies such as ThermoFisher, Abcam and Abnova. These methods use sample volumes of about 100μL and have a sensitivity of about 50-100 pg/mL making them to fail dramatically when low abundant biomarkers have to be determined in body fluids for early diagnosis. Numerous nonmainstream approaches have been proposed in the scientific literature for improving such sensitivity. We cannot be exhaustive here and we cite some of them as examples: Surface Plasmon Resonance (SPR); bio bar code assay; plasmonic ELISA; digital ELISA; MALDI-TOF mass spectrometry. Most of them have been tested also for determining AD-related biomarkers. Even though they present a considerable improvement of sensitivity, they all have a limited success for routine clinical practice for the following main reasons. 1) Their common denominator is the use of highly expensive technologies based for example on laser ionization, Au thin coatings, magnetic and Au nanoparticles, or nanowell platforms, that are accessible only to highly specialized labs. 2) They all require time consuming and highly critical sample preparation that goes well beyond the routine practice, thus adding non-negligible uncertainties to the assay and the need of highly skilled personnel. 3) They all require sample volumes of at least tens of μL, thus preventing applications with very little volumes. All of these drawbacks hinder mass production and accessibility to routine clinical tests.
The droplet-split-and-stack (DSS)
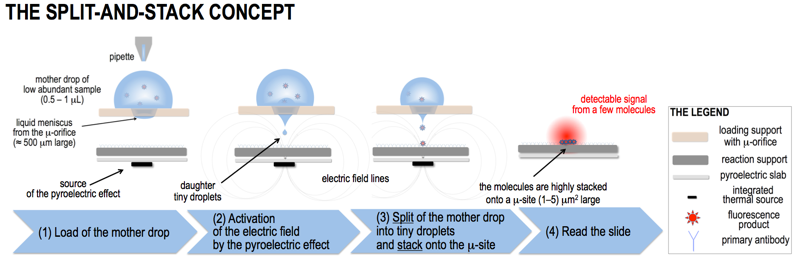
We propose here for the first time to stack droplets of blood sample for forcing the reaction between the protein biomarker and the corresponding antibody into volumes at sub-μL levels to overcome the diffusion limits, maximise the probability they encounter each other, stack the resulting immunocomplex product into an extremely confined site and hence increase the fluorescence (FL) signal per unit area, with the ambition of reaching a sensitivity much lower than 1 pg/mL. The way to achieve this breakthrough is explained in the following. FIG.1 shows the concept of DSS technology (not to scale). A mother drop of the sample (1L) with low abundant biomarker molecules (dots in the scheme and also called “antigens” for simplicity) is loaded on the micro-engineered μ-orifice (∼500μm large) of the loading support to form a thin liquid meniscus on the opposite face. A slab that we call reaction support is functionalized by immobilizing the appropriate antibody (high density and high affinity with the antigen in sample), and faces the μ-orifice. A pyroelectric slab, functionalized by a point-wise thermal source aligned with the μ-orifice, works as source of the pyro-electrohydrodynamic jet (p-jet) that triggers the rapid dispensing of tiny daughter droplets (‘split’) onto the same site (‘μ-site’) of the reaction support. The volume of the droplets depends mainly on the meniscus shape of the mother drop and on the electric field intensity and can be down to 10-6 pL. For simplicity, the antigen molecules in FIG.1 are fluorescent but, in realty, they are labelled by secondary reactions after stacking.
The pyro-electrohydrodynamic jet
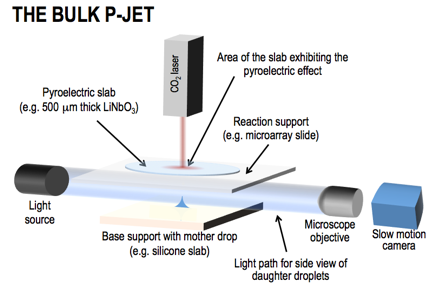
The pyro-electrohydrodynamic-jet (p-jet) is a radically innovative method that uses the pyroelectric effect for producing tiny droplets (down to about 10-6pL), developed for the first time by the CNR-ISASI team. In fact, the pyroelectric effect is well known only for energy detectors, intruder detectors, fire prevention, energy conservation, pollution monitoring, and thermal imaging. The p-jet draws daughter droplets directly from the meniscus of the mother drop pipetted by the operator on the loading support (e.g. 1μL), producing the smallest volumes ever seen nowadays by an agile nozzle- and electrode-free system. Commercial and frontier systems produce droplets with larger or even comparable volumes but all with the common issue of using a nozzle. This leads them to encounter big issues related to sample canalization, pressure regulation, large dead volumes, cross contamination and clogging. FIG.2 shows the scheme of the bulk p-jet (not to scale). It is a non-integrated and non-automated bench setup currently mounted in the lab of the CNR team. A pyroelectric slab is able to generate a charge density on the surface when subjected to a transient and slight temperature (T) gradient (few degrees with respect to room T). The resulting electric field causes the ejection of tiny droplets from the mother drop. For deeper understanding of both the pyroelectric effect and the electro-hydrodynamic droplet ejection we refer to valuable literature for reasons of space. The unique nozzle- and electrode-free character of p-jet favours its integrability into a cost-effective super-sensor.